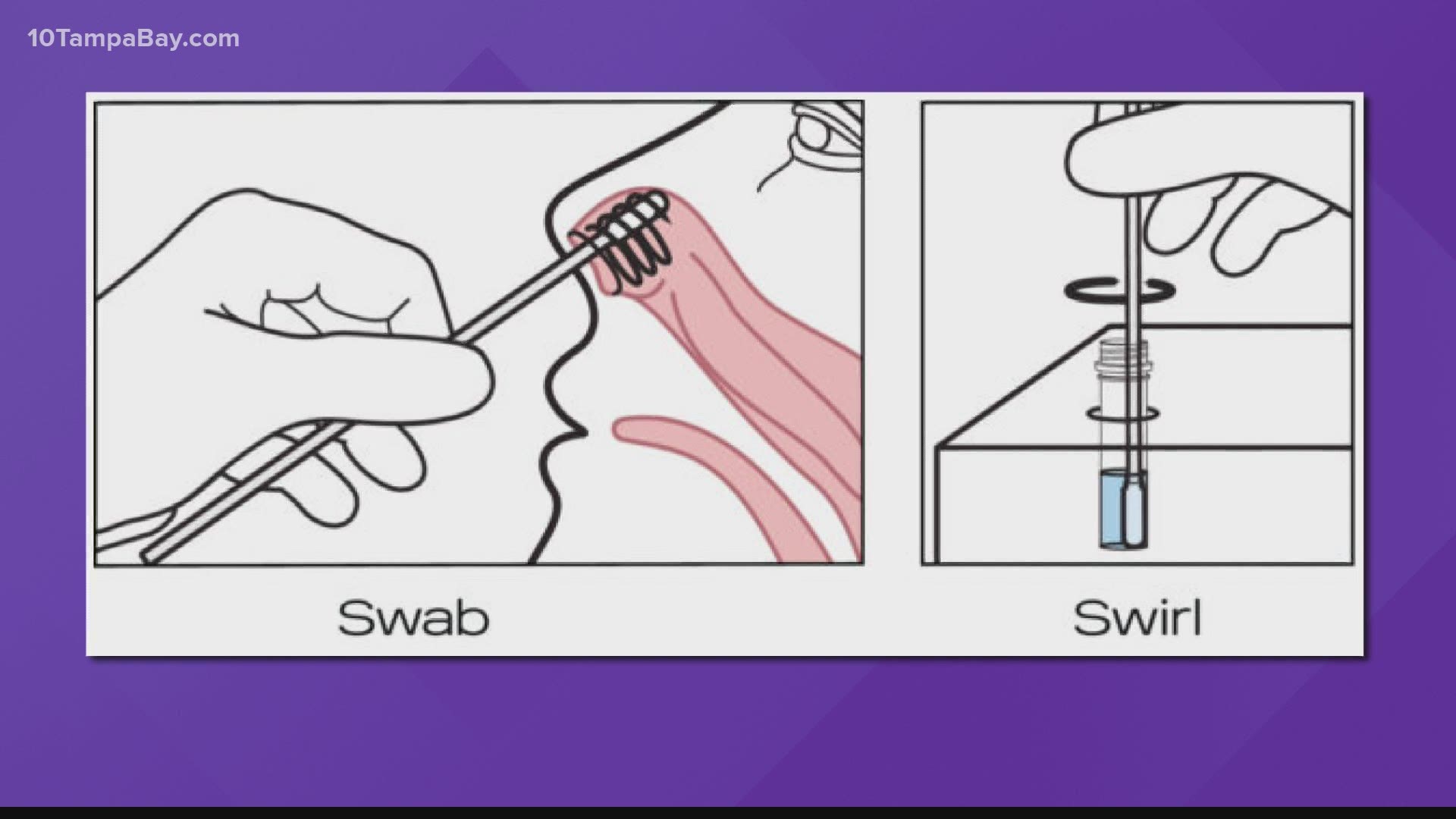
TAMPA, Fla — The FDA has issued an emergency use authorization for an at-home COVID-19 test kit. Health experts called the announcement exciting since it provides another tool Americans can use in the fight against coronavirus.
The test is called: Quidel QuickVue At-Home COVID-19 test.
It's for prescription home use with nasal swabs to collect a sample for yourself. The kit is meant to be used by people age 14 and older or those 8 and older with swabs collected by an adult. And you don't have to send that sample to the lab for analysis.
Dr. Jill Roberts with USF Health says since you don't go to a lab the state loses that data, but here's why she says it's OK.
"What I think the home test is gonna pick up is the people who weren't gonna get tested because it was too much work. And so honestly, we don't lose any data that we would have had anyway," Dr. Roberts said. "The best thing that comes from this, from a public health perspective, is the person finds out they're COVID positive and hopefully quarantines."
According to Dr. Roberts, the test could also help identify post-COVID syndrome. Meaning, if someone who tested positive is still having issues months later, they can call their doctor and get the assistance needed.
- Gov. Ron DeSantis delivers State of the State address
- Evictions loom for Tampa Bay families despite moratorium
- As vaccinations ramp up, COVID testing is down. That could be a problem, doctors say
- Are you eligible? Health insurance sign-ups are back open
- Iceberg larger than New York City breaks off Antarctica
- Family welcomed back to Florida with seven-foot gator in garage
►Breaking news and weather alerts: Get the free 10 Tampa Bay app
►Stay In the Know! Sign up now for the Brightside Blend Newsletter